Martian rust
An iron nail turns red in a solution of copper salt


Reagents
Safety
- Wear protective gloves.
- Do not allow chemicals to come into contact with the eyes or mouth.
- Keep young children, animals and those not wearing eye protection away from the experimental area.
- Store this experimental set out of reach of children under 12 years of age.
- Clean all equipment after use.
- Make sure that all containers are fully closed and properly stored after use.
- Ensure that all empty containers are disposed of properly.
- Do not use any equipment which has not been supplied with the set or recommended in the instructions for use.
- Do not replace foodstuffs in original container. Dispose of immediately.
- In case of eye contact: Wash out eye with plenty of water, holding eye open if necessary. Seek immediate medical advice.
- If swallowed: Wash out mouth with water, drink some fresh water. Do not induce vomiting. Seek immediate medical advice.
- In case of inhalation: Remove person to fresh air.
- In case of skin contact and burns: Wash affected area with plenty of water for at least 10 minutes.
- In case of doubt, seek medical advice without delay. Take the chemical and its container with you.
- In case of injury always seek medical advice.
- The incorrect use of chemicals can cause injury and damage to health. Only carry out those experiments which are listed in the instructions.
- This experimental set is for use only by children over 12 years.
- Because children’s abilities vary so much, even within age groups, supervising adults should exercise discretion as to which experiments are suitable and safe for them. The instructions should enable supervisors to assess any experiment to establish its suitability for a particular child.
- The supervising adult should discuss the warnings and safety information with the child or children before commencing the experiments. Particular attention should be paid to the safe handling of acids, alkalis and flammable liquids.
- The area surrounding the experiment should be kept clear of any obstructions and away from the storage of food. It should be well lit and ventilated and close to a water supply. A solid table with a heat resistant top should be provided
- Substances in non-reclosable packaging should be used up (completely) during the course of one experiment, i.e. after opening the package.
FAQ and troubleshooting
The nail is covered with something black. Is something wrong?
If the nail you put in the copper sulfate solution turned black instead of becoming “Martian” (red), don’t take it out the solution. A very thin black layer is also formed by copper. Wait a bit and you will see red copper depositing on the nail.
If you took the nail out of the solution, carefully remove the black deposit with a paper towel. Put the nail back into the solution and wait 20-30 seconds more.
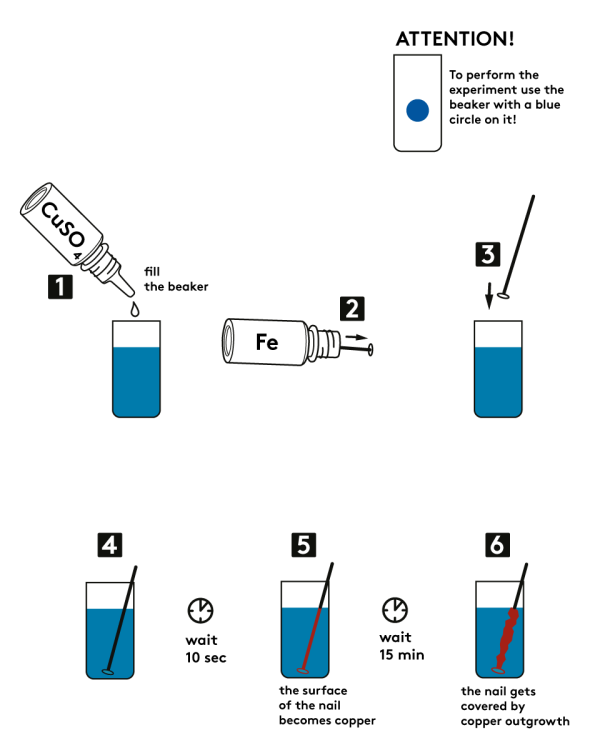
Step-by-step instructions
- Take a disposable cup marked with a blue circle. Fill it with 0.5M copper sulfate CuSO4 solution. Use no more than one bottle of it (7 mL).
- Take an iron nail from the bottle labeled “Fe”.
- Place the nail into the cup with copper sulfate solution.
- Wait for about 10 seconds. The nail surface covers with copper – it turns red. Who knows, Martian rust might look just the same!
- Wait for 15 – 20 minutes more. The nail will be covered with copper crust.
Expected result
If the iron nail is placed briefly into the CuSO4 solution (≈ 10 seconds), it will be covered with a thin layer of red copper. If the nail is left in the CuSO4 solution for a long time, the nail becomes covered with a loose, spongy build-up of copper.
Disposal
Put the iron filings into the copper sulfate solution. Wait for 1 hour. After the blue solution turns clear, pour the liquid down the drain. Dispose of the experiment residues along with your regular household trash.
Scientific description
Why does the nail turn red?
The nail turns red because it is covered with a thin layer of copper. Iron from the surface of the nail goes into solution and the copper from the solution forms a thin layer on the nail's surface. The copper in solution is in the form of the positively charged ion Cu2+. This means that the copper is missing two electrons. Each electron carries a negative charge. To turn into an uncharged metal (Cu0), the copper takes its missing electrons from the iron of the iron nail.
Why does copper form on the surface of the nail?
Copper metal forms on the surface of the nail because copper is a less active metal than iron. The activity of a metal relates to its ability to donate electrons and become a positively charged ion. The more active a metal is, the more easily it can donate electrons and the more it tends to become an ion. In this case, the iron in the nail becomes an ion by giving up its electrons to the copper in the solution. Copper accepts these electrons and turns into its metal form.
Why is iron more active than copper?
To measure the activity of a metal, chemists use a special term, known as reduction potential or redox potential. Metals with the highest redox potential are the best reducing agents, which means they readily donate their electrons. Without going into the details of how redox potentials are calculated, we can put metals in order of decreasing redox potential value, or decreasing activity. The metals with the highest redox potentials are at the top of the list, which is also called a reactivity series:
- K (potassium)
- Ca (calcium)
- Li (lithium)
- Na (sodium)
- Mg (magnesium)
These metals have just one or two electrons and they part easily with them.
However, precious metals are not very active at all and do not easily give up their electrons. Therefore, the following metals are at the end of the reactivity series:
- Cu (copper)
- Hg (mercury)
- Ag (silver)
- Au (gold)
In the reactivity series, iron is in the middle between the highly active metals and the unreactive precious metals. Therefore, if copper and iron are together, it is the iron, which is more active, that gives the electron to copper. Iron has a larger redox potential than copper. Put simply, there are three main groups of metal activity: the elements in group 1 and 2 of the periodic table (the first two columns on the left) are the most active; the precious metals (for example, gold and silver are in group 11) are the least active; and all other metals are in between.
Follow up
What can we use instead of an iron nail?
Try carrying out the experiment with a piece of aluminum wire, a shiny metal screw, and a dark gray matt screw. Which materials have you succeeded with, and which failed the experiment? Do you have any suggestions why? Please share the results of your experiments with us on Facebook (https://www.facebook.com/melscience): let’s discuss them together!
That’s interesting!
Why does the blue solution become greenish?
The blue color of the copper sulfate solution comes from the copper ions. During the reaction the copper ions leave the solution to become copper metal on the surface of the nail. Similarly, the iron metal of the nail moves into solution as iron ions that have a 2+ charge. It is these iron ions that give a greenish color to the solution.
Why do I need to dispose of the copper solution in a special way?
Copper solutions are dangerous to aquatic organisms. Therefore, to dispose of copper in accordance with European safety standards, we need to transform copper ions into metal form. Iron in solution is much less dangerous than copper.